CARBON
AND ITS COMPOUNDS.
The chemical substances which consist
of carbon are called carbon compounds. the compounds which have single bonds
are called saturated compounds. Compounds of carbon with double bonds and
triple bonds are called unsaturated compounds.
Note: Double bond contains 1 sigma bond and 1 pie
bond.
Triple
bond contains 1 sigma bond and 2 pie bonds.
Ionic Bond.
The electrostatic force of attraction
between the oppositely charged ions of the atom holds the compound together
with the bond called an ionic bond. Ionic bond involves the transfer of valence
electrons, between a metal and a nonmetal. Ionic compounds are crystalline
solids (made of ions) that possess high melting and boiling points. They
conduct electricity in a solution and molten state. They are soluble in water
and polar solvents.
Covalent Bond.
The bond which is formed when pairs
of electrons are shared between two atoms is called a covalent bond. It is
usually formed between two same non-metallic atoms or between non-metallic
atoms with similar electronegativity. Such compounds are called diatoms.
Covalent
Bonding.
If the carbon atom loses four of its
valence electrons, to attain its nearest stable electronic configuration i.e, a
huge amount of energy is involved. C4+ ion hence formed will be
highly unstable due to the presence of six protons and two electrons.
If the carbon atom gains four electrons to get the
nearest electronic configuration of the noble gas, Ne, C4− ion will be formed. But again, a huge amount of
energy is required for C4+ ions it is difficult for 6 protons to
hold 10 electrons. Hence, to satisfy its tetravalency, carbon shares all four
of its valence electrons and forms covalent bonds.
Lewis Dot
Structure.
Lewis structures are also known as
Lewis’s dot structures or electron dot structures.
These are diagrams with the element’s symbol
in the centre. The dots around it represent the valence electrons of the
element.
Lewis structures of elements with atomic numbers 6,
7, 8.
Covalent Bonding in H2, N2,
and O2.
Formation
of a single bond in a hydrogen molecule:
Hydrogen atomic number is 1 and its electronic
configuration is 1s1. Each hydrogen atom has a single electron in
the valence shell. It requires one more to attain the nearest noble gas
configuration (He). Hence both atoms share one electron each to form a single
bond. It is represented by a single line between the two atoms.
Formation of a double bond in an
oxygen molecule:
The
oxygen atomic number is 8 and its electronic configuration is 1s2,2s2,2p4.
Each oxygen atom has six electrons in the valence shell (2, 6). It requires two
electrons to attain the nearest noble gas configuration (Ne). Hence, both atoms
share two electrons each and form a double bond. It is represented by double
lines between the two atoms.
Formation of a triple bond in a
nitrogen molecule:
Nitrogen
atomic number is 7 and its electronic configuration is 1s2,2s2,2p3.
Each nitrogen atom has five electrons in the valence shell (2, 5). It requires
three electrons to attain the nearest noble gas configuration (Ne). Hence, both
atoms share three electrons each and form a triple bond. It is represented by
triple lines between the two atoms.
Single, Double, and Triple Bonds and Their Strengths.
Bond
strength:
The bond strength of a
bond is determined based on the amount of energy required to break the bond. It
signifies that the energy required to break three bonds is higher than that for
two bonds or a single bond.
Order of Bond: Triple bond>double bond>single bond
Bond
length:
Bond length is determined
by the distance between nuclei of the two atoms in a bond. The distance between
the nuclei of two atoms is the least when they are triple-bonded.
Order Of Bond Length: Triple bond<double bond<single bond.
Covalent
Bonding of N, O with H and Polarity.
Ammonia.
In ammonia (NH3), the
three hydrogen atoms share one electron each with the nitrogen atom and form
three covalent bonds. Ammonia has one lone pair. All three N-H covalent bonds
are polar. It forms a shape of trigonal pyramidal and the bond angle is 107’.
N atom is more electronegative than the H atom.
Thus, the shared pair of electrons lies more towards N atom which causes the N
atom to acquire a slightly negative charge and H atom a slightly positive
charge.
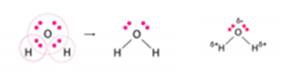
Water.
In water (H2O), the two
hydrogen atoms share one electron each with the oxygen atom and form two
covalent bonds. Water has two lone pairs of electrons. It forms an angular (or)
V shape whose bond angle is 104.5’.
The two O-H covalent bonds are polar in nature, a
hydrogen bond is formed between 2 molecules of water. O atom is more electronegative than the H atom.
Thus, the shared pair of electrons lies more towards O atom which causes the O
atom to acquire a slightly negative charge and H atom a slightly positive
charge.
Covalent
Bonding in Carbon.
A methane molecule (CH4)
is formed when four electrons of carbon are shared with four hydrogen atoms as
shown below. It forms a shape tetrahedral with a bond angle of 109.5’ and a
bond length is 108.7pm.
Catenation occurs most readily with
carbon due to its small size, electronic configuration, and unique strength of
carbon-carbon bonds. Tetravalency, catenation and the tendency to form multiple
bonds with other atoms account for the formation of innumerable carbon
compounds.
Catenation.
Catenation is the property of an
element that forms covalent bonds with the other atoms of the same element to
form a series of straight or branched chains and rings of different sizes.
Examples; carbon, sulphur, and silicon.
Carbon.
Organic compounds are formed by the
combination of Hydrogen, oxygen, carbon, few elements. They are significantly
more numerous than inorganic compounds that do not form bonds. Carbon has
tetravalency, and Catenation It forms single, double, and triple bonds
demonstrates its versatility. It forms straight chains, branching chains, and
rings when joined to other carbon atoms.
Carbon is a chemical element whose
symbol C and its atomic number 6 which is a versatile element that can be found
in a wide variety of chemical combinations. The property of carbon elements due
to which its atom can join one another to form long carbon chains is called
catenation.
Melting point, Boiling point, and Electrical Conductivity of covalent
compounds.
They are molecular compounds that are present as gases, liquids or solids. They have weak intermolecular forces, with low melting and boiling points. They possess poor electrical conductors in all phases specifically in solution or molten state. They are mostly soluble in nonpolar liquids.
Allotropes of Carbon.
The phenomenon of the existence of
the same element in different physical forms with similar chemical properties
is known as allotropy.
Example: carbon, Sulphur, phosphorus, etc.
- Amorphous
allotropes of carbon include coal, coke, charcoal, lamp black.
- Crystalline
allotropes of carbon include diamond, graphite, and, fullerene.
Diamond.
Diamond has a regular tetrahedral
geometry because each carbon is connected to four neighboring carbon atoms with
a single covalent bond, which forms in a single unit of a crystal. These
crystal units lie in different planes and are connected to each other, forms a
rigid three-dimensional cubic pattern of the diamond.
- Diamond
is a good conductor of heat.
- They
are poor conductor of electricity.
- They
possess very high refractive index of 2.5.
- They have high density of 3.5g/cc.
Graphite.
In graphite, each carbon atom is
bonded covalently to three other carbon atoms, leaving each carbon atom with
one free valency. This arrangement results in hexagonal rings in a single plane
and such rings are stacked over each other through weak Van der Waals forces.
Graphite is a good conductor of electricity which
shows a soft and slippery structure whose a density of 2.25 g/cc.
C60.
It is the most common naturally occurring fullerene
also known as Buckminsterfullerene, which is the very popular
and stable form of the known fullerenes. It is found in small quantities in
soot. It consists of 60 carbon atoms arranged in 12 pentagons and 20 hexagons,
like in a soccer ball.
S8
In its native state, sulphur show catenation of up to 8 atoms in the form of S8 molecule. It has a puckered ring structure.













Comments
Post a Comment